Kanker is een van de grootste uitdagingen waar we voor staan!
13 Miljoen mensen zullen sterven aan kanker in 2035 (Global Cancer Observatory), 40% van de mensen zal in zijn leven kanker krijgen (ACS). De wereldwijde kosten van kanker voor de samenleving lopen op tot 1,2 Miljard dollar (WH0).
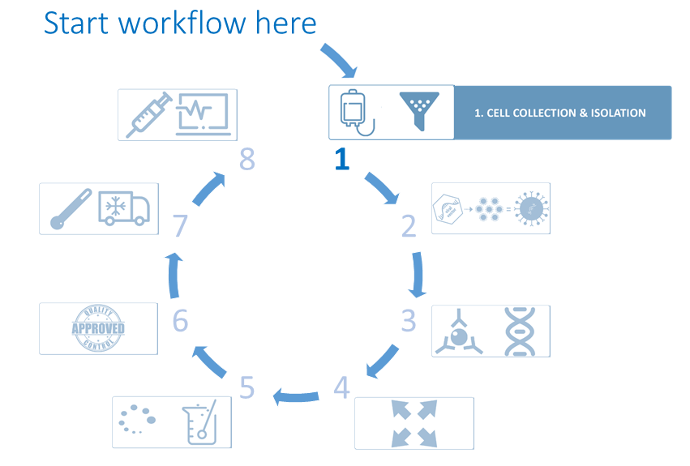
De ontwikkeling van immunologische biofarmaceutische producten is de laatste jaren opvallend toegenomen en heeft een duidelijke bijdrage geleverd aan de menselijke gezondheid. De workflow die hier en op de bijbehorende pagina's wordt gepresenteerd, beschrijft de behoeften met betrekking tot de ontwikkeling en productieprocessen van CAR-T-cellen (*1).
Immunotherapeutische benaderingen
Antilichamen zijn de belangrijkste entiteit in immuuntherapie, terwijl chimere antigeen receptor T-celtherapieën de komst zijn van een nieuwe strategie op dit gebied. Er zijn veel potentiële immuuntherapeutische benaderingen, waaronder celgebaseerde strategieën zoals adoptieve celoverdracht, receptorpadweggebaseerde strategieën zoals checkpointremming en agentgebaseerde benaderingen zoals antilichaamtherapie.
Genetische manipulatie kan worden opgenomen in al deze benaderingen. Genbewerking kan worden gebruikt om receptorexpressieniveaus te moduleren, de productie van bepaalde moleculen te induceren, cellulaire fenotypen te veranderen of de algehele intensiteit van de immuunrespons te wijzigen.
Vandaag de dag worden celtherapieën voortdurend ontwikkeld en verbeterd en bieden ze nieuwe opties voor kankerpatiënten. Celtherapieën worden momenteel geëvalueerd, zowel alleen als in combinatie met andere behandelingen, in klinische studies voor verschillende soorten kanker. Adoptieve celtherapie (ACT), ook bekend als cellulaire immuuntherapie, is een vorm van behandeling die gebruik maakt van de cellen van ons immuunsysteem om kanker te elimineren. Bij sommige van deze benaderingen worden onze eigen immuuncellen rechtstreeks geïsoleerd en wordt hun aantal eenvoudig uitgebreid, terwijl bij andere benaderingen onze immuuncellen genetisch worden gemanipuleerd (via gentherapie) om hun kankerbestrijdende capaciteiten te vergroten.
De gebruikte cellen kunnen afkomstig zijn van 2 verschillende bronnen, afhankelijk van het gevolgde proces. Bij autologe transplantaties zijn ze afkomstig van dezelfde persoon die het transplantaat zal krijgen, dus de patiënt is zijn eigen donor. Omgekeerd zijn allogene transplantatiecellen afkomstig van een andere persoon dan de patiënt, of het nu een verwante of niet-verwante donor is.
Ons immuunsysteem is in staat om cellen die geïnfecteerd of beschadigd zijn geraakt te herkennen en te elimineren, evenals cellen die kankerachtig zijn geworden.
Inleiding & overzicht van immunotherapie
Bespreking van de basisprincipes van immunotherapie en de verwante middelen en technieken die vaak worden gebruikt.
Maakt immunotherapie zijn beloften waar?
Bekijk dit webinar over de vooruitgang en prestaties van immunotherapie, gepresenteerd door Jill O'Donnell-Tormey en Alex Y. Huang
In het geval van kanker zijn immuuncellen die bekend staan als killer T-cellen bijzonder krachtig tegen kanker vanwege hun vermogen om zich te binden aan markers die bekend staan als antigenen op het oppervlak van kankercellen. Cellulaire immuuntherapieën maken gebruik van dit natuurlijke vermogen en kunnen op verschillende manieren worden ingezet:
Tumor-Infiltrerende Lymfocyt (TIL)-therapie, engineered T Cell Receptor (TCR)-therapie, chimere antigen receptor (CAR)-Tceltherapie, Natural Killer (NK)-celtherapie.
Door de hulpmiddelen van de synthetische biologie, zoals CAR's en CRISPR/Cas9 (*4) hebben we een ongekende kans om T-cellen optimaal te programmeren en adoptieve immuuntherapie te verbeteren voor de meeste, zo niet alle toekomstige patiënten.
De somatische mutaties die kankercellen hebben verworven, kunnen door het immuunsysteem worden herkend als 'niet-zelf' en zijn in staat om een immuunrespons op te wekken die zich selectief richt op tumorcellen en deze verwijdert. Er zijn een aantal stappen nodig om de peptiden aan het immuunsysteem te laten zien en elk van deze processen heeft optimale omstandigheden waaronder ze plaatsvinden. Ondanks het grote aantal potentiële neoantigenen (*5) in sommige kankers met een hoge mutatielast, is slechts een fractie in staat om uiteindelijk een immuunrespons op te wekken. Met de verbetering van de moleculaire en in silico mogelijkheden in de afgelopen jaren, is het aantal geïdentificeerde immunogene neoantigenen aanzienlijk toegenomen. Hoe groter het aantal geverifieerde neoantigenen, hoe beter de getrainde in silico voorspellingstools in staat zijn om betrouwbaar diegenen te identificeren die klinisch nut kunnen hebben.
Analis biedt ongeëvenaarde expertise ter ondersteuning van de ontwikkeling van kanker immuuntherapieën, waaronder: Adoptieve Cel Therapieën (ACT), CAR T-Cel Therapieën (*1), Immune Checkpoint Inhibitor (*2), Gentherapieën (*3), Vaccins. We willen graag de volgende generatie celtherapieën bevorderen door te onderzoeken hoe we testen robuust kunnen valideren en de karakterisering van celimmunotherapieanalyses kunnen bevorderen om veilige, effectieve en hoogwaardige celtherapieproducten te garanderen.
CAR-T celtherapie: Inleiding & overzicht
Video over de introductie van CAR-T celtherapie als onderdeel van immuuntherapie.
Recente vooruitgang in immuuntherapie - Cellen sturen om ziekte aan te pakken
Bekijk deze webinar gepresenteerd door Leena Gandhi MDR PhD over de recente vooruitgang in immuuntherapie met een focus op het sturen van cellen om ziekte aan te pakken.
Definities
(*1) Chimeric Antigen Receptor T-cellen (CAR-T-cellen)
Adoptieve celtherapie met behulp van chimere antigeen receptor T-cellen (CAR-T-cellen) is een veelbelovende strategie voor immuuntherapie tegen kanker en heeft de laatste jaren een snelle ontwikkeling doorgemaakt. CAR-T-cellen zijn genetisch gemodificeerde T-cellen (van de patiënt zelf of van een donor) die een chimeer antigeen tot expressie brengen dat specifiek tumorspecifieke antigenen op het oppervlak van tumorcellen kan herkennen en vervolgens effectief tumorcellen kan doden. CAR-T-therapieën worden beschouwd als geneesmiddelen voor geavanceerde therapie (ATMPs) in Europa, en meer specifiek geneesmiddelen voor gentherapie (GTMPs).
(*2) Immune Checkpoint Inhibitors ;
Geneesmiddelen met de naam Immune Checkpoint Inhibitors werken door een natuurlijke rem op uw immuunsysteem los te laten, zodat immuuncellen, T-cellen genaamd, tumoren herkennen en aanvallen.
Deze therapie wordt ook wel immune checkpointblokkade genoemd, omdat het molecuul dat als rem op immuuncellen werkt - het checkpoint - door het geneesmiddel wordt geblokkeerd. Dit voorkomt dat het "uit"-signaal wordt verzonden, waardoor de T-cellen kankercellen kunnen doden.
(*3) Gentherapie ;
Gentherapie bij mensen is gericht op het wijzigen of manipuleren van de expressie van een gen of het veranderen van de biologische eigenschappen van levende cellen voor therapeutisch gebruik. Gentherapie is een techniek die de genen van een persoon modificeert om ziekten te behandelen of te genezen.
Gentherapieën kunnen via verschillende mechanismen werken:
- Een ziekmakend gen vervangen door een gezonde kopie van het gen
- Een ziekteveroorzakend gen dat niet goed functioneert inactiveren
- Het introduceren van een nieuw of gewijzigd gen in het lichaam om een ziekte te helpen behandelen
- Gebruik van RNA-interferentie of mRNA-technologie om genexpressie te reguleren
Gentherapieproducten worden onderzocht voor de behandeling van ziekten zoals kanker, genetische ziekten en infectieziekten. Er zijn verschillende soorten gentherapieproducten, waaronder:
- Plasmide DNA: Cirkelvormige DNA-moleculen kunnen genetisch gemanipuleerd worden om therapeutische genen in menselijke cellen te brengen.
- Virale vectoren: Virussen hebben een natuurlijk vermogen om genetisch materiaal in cellen te brengen en daarom zijn sommige gentherapieproducten afgeleid van virussen. Zodra virussen zijn aangepast om hun vermogen om infectieziekten te veroorzaken te verwijderen, kunnen deze aangepaste virussen worden gebruikt als vectoren (voertuigen) om therapeutische genen in menselijke cellen te brengen.
- Bacteriële vectoren: Bacteriën kunnen worden gemodificeerd om te voorkomen dat ze infectieziekten veroorzaken en vervolgens worden gebruikt als vectoren (voertuigen) om therapeutische genen in menselijke weefsels te brengen.
- Technologie voor het bewerken van menselijke genen: Het doel van genbewerking is om schadelijke genen te verstoren of gemuteerde genen te repareren.
- RNA gebaseerde therapieën: deze omvatten het gebruik van RNA, inclusief mRNA, om genexpressie te reguleren of om therapeutische eiwitten te coderen.
- Van de patiënt afgeleide cellulaire gentherapieproducten: Cellen worden bij de patiënt weggehaald, genetisch gemodificeerd (vaak met behulp van een virale vector) en vervolgens teruggestuurd naar de patiënt.
(*4) Clustered regulatory interspaced short palindromic repeat/CRISPR-geassocieerd eiwit 9 (CRISPR/Cas9)
DeCRISPR/Cas9-technologie(clustered regulatory interspaced short palindromic repeat/CRISPR-associated protein 9) houdt een enorme belofte in voor vooruitgang op dit gebied vanwege zijn flexibiliteit, eenvoud, hoge efficiëntie en multiplexing bij het nauwkeurig bewerken van het genoom. CRISPR-systemen splijten dubbelstrengs DNA, waardoor een herstelmechanisme van de gastheer in werking wordt gesteld dat het gen kan inactiveren door de introductie van insertie-/deletiemutaties op de splitsingsplaats. Het echte nut van CRISPR is echter dat deze splitsing kan worden gestuurd met behulp van geleider-RNA's, waardoor wetenschappers specifieke genen van belang kunnen inactiveren.
Ondanks het succes van CAR-T celtherapieën is een aantal patiënten niet in staat deze therapie te ondergaan vanwege onvoldoende T-celaantallen of snelle ziekteprogressie. Bovendien is het gebrek aan respons op behandeling met CAR-T-cellen in sommige gevallen te wijten aan intrinsieke autologe T-celdefecten en/of het onvermogen van deze cellen om optimaal te functioneren in een sterk immunosuppressieve tumormicro-omgeving. Er zijn recente pogingen beschreven om deze beperkingen te overwinnen met behulp van CRISPR/Cas9-technologie, met als doel de werkzaamheid te verbeteren en de beschikbaarheid van CAR-gebaseerde therapieën te vergroten. Door het combineren van de gereedschappen van de synthetische biologie, zoals CAR's en CRISPR/Cas9, bestaat er een ongekende mogelijkheid om T-cellen optimaal te programmeren en adoptieve immuuntherapie voor de meeste, zo niet alle toekomstige patiënten te verbeteren.
Bekijk voor meer informatie over hoe CRISPR kan worden gebruikt in immuuntherapie de korte video in Step 2. Vector Design & Production.
(*5) Neoantigeen
Een Neoantigen is een nieuw eiwit dat zich vormt op kankercellen wanneer bepaalde mutaties optreden in tumor-DNA. Het kan een belangrijke rol spelen bij het helpen van het lichaam om een immuunreactie tegen kankercellen te maken.
Sources :
- Waldman, A.D., Fritz, J.M. & Lenardo, M.J. (2020). A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 20, 651–668 (2020). https://doi.org/10.1038/s41577-020-0306-5
-
Salas-Mckee, J., Kong, W., Gladney, W. L., Jadlowsky, J. K., Plesa, G., Davis, M. M., & Fraietta, J. A. (2019). CRISPR/Cas9-gebaseerde genoombewerking in het tijdperk van CAR T cel immunotherapie. Humane vaccins & immunotherapeutica, 15(5), 1126-1132. https://doi.org/10.1080/21645515.2019.1571893 -
Hutchison, S., & Pritchard, A. L. (2018). Het identificeren van neoantigenen voor gebruik in immunotherapie. Mammalian genome : official journal of the International Mammalian Genome Society, 29(11-12), 714-730. https://doi.org/10.1007/s00335-018-9771-6 - Onderzoek en informatie over immunotherapie - Beckman Coulter