The success of using viral vectors to deliver a molecular payload into a cell or replace defective genes with functional ones is an inflection point in the future of modern medicine.
Vector production is a full workflow including :
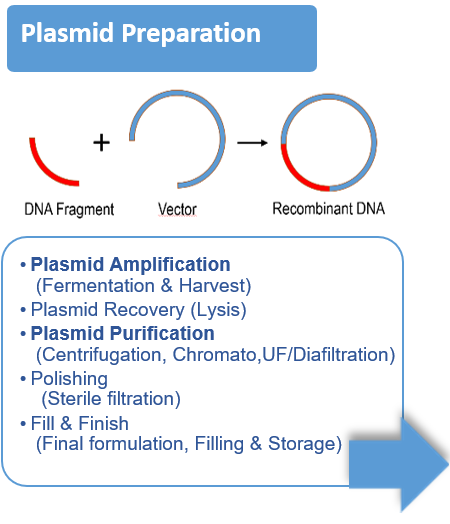
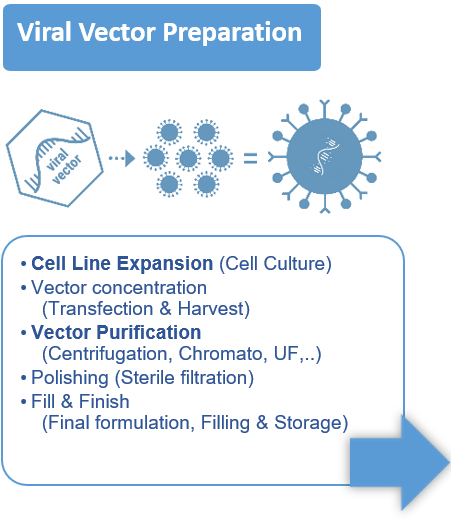
- Vector design, cell expansion, transfection / infection, cell lysis, harvest / clarification, concentration, capture, polishing, formulation, sterile filtration.
- Most of the time the vector is a virus (AAV, Lentivirus, ..), a plasmid, but it can also be an Extracellular Vesicle.


Your challenges in vector production
- Virus or Pathogen manipulations in safe conditions?
- Is your Capsid Load characterization method robust, reliable and validated?
- How can the purity of the vector be increased?
- Is your cell count and viability analysis method easy and reliable?
Ask our experts
Our solutions for your vector Workflow
- Air sampling for viable particle monitoring in clean rooms
- Automated liquid handling, Automated non contact liquid handing
- Biomass by spectrophotometry
- Bioreactor
- Bioreactor automated cell counter & viability analyzers
- Bioreactor media health bioanalyzer
- Biosafety Cabinet
- Capillary Electrophoresis (CE) for plasmid purity, viral protein analysis, empty/full capsid quantification, genome integrity
- Cell Harvesting
- Centrifuges, Centrifuge Biosafe labwares and accessoires
- CO2 Incubator, Shaker incubator
- Disinfection
- Elisa Manual methods with microplate reader and microplate washer
- Elisa Automated solutions, 10 things to consider for ELISA automation
- Flow cytometer
- Fridges, freezers and deep-freezers +4°C to -80°C, Liquid N2
- Cryogenic preservation
- Imaging for Western blot
- Incubator shaker
- PCR Setup, PCR Cabinet
- Pipet
- Preparative Ultracentrifuge, Analytical Ultracentrifuge (AUC)
- Viral Vector Capside Load by Analytical Ultracentrifugation (AUC) or by Capillary Electrophoresis (CE)
- Viral Vector Size, shape and structure by Analytical
- Ultracentrifugation (AUC), by Capillary Electrophoresis (CE) or by Laser diffraction (LS)
- Steam sterilization
-
Water bath
As a quick recap, gene therapy typically involves inserting genetic material into cells via a harmless viral vector that “infects” the cell and delivers the genetic payload. Analytical methods are needed for raw material testing, in-process control and for demonstrating the quality of the Viral Vector batch. GMP Viral Vector batches that are manufactured for clinical trials must comply with an increasing number of global regulations. Safety, identity, strength, purity and quality among the items that need to be demonstrated.
One of the most popular vectors for packaging is the adeno-associated virus (AAV), which has two key benefits: efficiency as a gene delivery vector and low pathogenicity. The virus has been modified from the wild type to optimize its efficiency as a therapeutic gene carrier and minimize its potential to cause disease. Recombinant AAV is the leading platform for gene therapy today.
Creating safe products that can be scaled up – and retain their safety – is key. The important element in manufacturing gene therapy products is purity, which refers to the efficiency of “packaging” for delivery into the cell. Poor packaging can lead to less effective therapy. And giving higher doses of therapy to offset poor packaging risks triggering an allergic reaction in the patient. The FDA has issued guidance for clinical trials that addresses the importance of this very issue.
All the principles developed here above are of course used in many other advanced therapy medicinal products (ATMPs) than immunotherapies. It is also important to highlight that following COVID19 period, the lipid nanoparticles(LNPs) are a more common carrier for genetic material like mRNA.
Development & Production of Viral Vectors:
Advances in Processes and Translation for Human Gene Therapy
Understand the role viral vectors play in modern gene research and how ultracentrifugation is used to produce these key delivery systems
Christine Le Bec - Analytical methods to measure empty & full AAV particles
Review around biophysical characterizing techniques and a focus on the use of analytical ultracentrifugation (AUC) for measuring empty and full AAV particles.
CRISPR for Immunotherapy: Introduction & Overview
Featured video on the introduction to CRISPR for immunotherapy.
Sources and references:
Hacein-Bey-Abina, S., Hauer, J., Lim, A., Picard, C., Wang, G. P., Berry, C. C., Martinache, C., Rieux-Laucat, F., Latour, S., Belohradsky, B. H., Leiva, L., Sorensen, R., Debré, M., Casanova, J. L., Blanche, S., Durandy, A., Bushman, F. D., Fischer, A., & Cavazzana-Calvo, M. (2010). Efficacy of gene therapy for X-linked severe combined immunodeficiency. The New England journal of medicine, 363(4), 355–364.
https://doi.org/10.1056/NEJMoa1000164
Kaeppel, C., Beattie, S. G., Fronza, R., van Logtenstein, R., Salmon, F., Schmidt, S., Wolf, S., Nowrouzi, A., Glimm, H., von Kalle, C., Petry, H., Gaudet, D., & Schmidt, M. (2013). A largely random AAV integration profile after LPLD gene therapy. Nature medicine, 19(7), 889–891.
https://doi.org/10.1038/nm.3230
Masat, E., Pavani, G., & Mingozzi, F. (2013). Humoral immunity to AAV vectors in gene therapy: challenges and potential solutions. Discovery medicine, 15(85), 379–389.
Mingozzi, F., & High, K. A. (2013). Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood, 122(1), 23–36.
https://doi.org/10.1182/blood-2013-01-306647
Joshi, P., Cervera, L., Ahmed, I., Kondratov, O., Zolotukhin, S., Schrag, J., Chahal, P. S., & Kamen, A. A. (2019). Achieving High-Yield Production of Functional AAV5 Gene Delivery Vectors via Fedbatch in an Insect Cell-One Baculovirus System. Molecular therapy. Methods & clinical development, 13, 279–289.
https://doi.org/10.1016/j.omtm.2019.02.003